Common Eye Ointment Found to Damage Glaucoma Implants
 Japanese research prompts urgent clinical guidance update after petrolatum-based products shown to cause device swelling and rupture. Eyecare professionals are being urged to avoid using petrolatum-based eye ointments on patients with glaucoma drainage implants, following research from Nagoya University that reveals these widely-used products can cause the devices to swell and potentially rupture.
Japanese research prompts urgent clinical guidance update after petrolatum-based products shown to cause device swelling and rupture. Eyecare professionals are being urged to avoid using petrolatum-based eye ointments on patients with glaucoma drainage implants, following research from Nagoya University that reveals these widely-used products can cause the devices to swell and potentially rupture.
The study, published in Graefe's Archive for Clinical and Experimental Ophthalmology, is the first to provide both clinical and experimental evidence that petrolatum-based ointments can seriously compromise the PRESERFLO® MicroShunt, a drainage device now used in more than 60 countries to manage glaucoma.
Critical finding for post-operative care
The research team, led by ophthalmologist Dr Ryo Tomita from Nagoya University Graduate School of Medicine, examined seven clinical cases alongside laboratory experiments to understand the interaction between the implant material and common post-operative treatments.
"Swollen MicroShunts can be structurally fragile," Dr Tomita said. "During surgery, I observed a rupture in a swollen MicroShunt. If more clinicians are aware of this risk, they will be able to prevent similar problems."
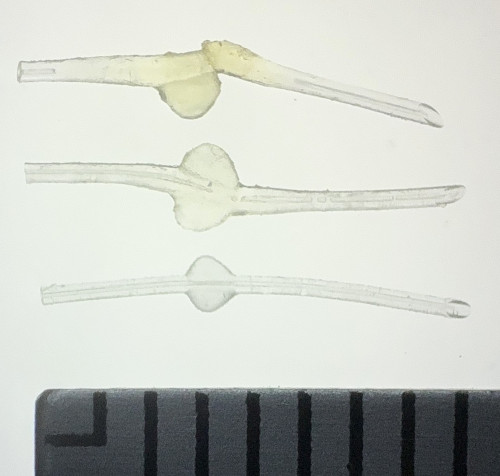
The findings revealed a stark pattern: all three patients whose exposed MicroShunts were treated with petrolatum-based ointment showed significant device swelling, with two implants rupturing. By contrast, devices that remained covered by the conjunctiva or weren't exposed to the ointment maintained their original structure.
Understanding the mechanism
The MicroShunt is manufactured from a styrenic thermoplastic elastomer (SIBS polymer), chosen for its biocompatibility and reduced inflammatory response. However, this material's high oil affinity makes it vulnerable to hydrocarbon-based substances.
Laboratory testing confirmed the clinical observations. After just 24 hours immersed in petrolatum-based ointment, the device's outer diameter increased to 1.44 times its original size. Chemical analysis showed oil components comprised approximately 45% of the implant's weight after one day, rising to 73% after three months.
Manufacturer warnings overlooked
While the MicroShunt manufacturer's instructions explicitly warn against direct contact with petrolatum-based materials, the research team found this precaution is not widely recognised or consistently followed in clinical practice.
The collaborative study brought together medical and engineering expertise, with Dr Tomita and colleagues from Nagoya University Hospital working alongside engineering researchers Dr Takato Kajita and Associate Professor Atsushi Noro.
"Our study found that commonly used medical materials can cause unexpected complications if their chemical properties and usage environments are not fully understood," Professor Noro stated. "From both medical and engineering perspectives, we emphasise the importance of understanding the chemical properties of medical materials and appropriately managing their usage environments."
Implications for practice
The researchers stress that clinicians should immediately cease using petrolatum-based ointments on patients with MicroShunt implants, particularly when the device is exposed outside the conjunctiva. Alternative post-operative treatments should be considered as standard care.
The team has also called for further research to determine whether swelling affects device performance even when rupture doesn't occur, a question with implications for the estimated 76 million people globally affected by glaucoma.
(photo credit: Ryo Tomita)